BEDFORD, Mass.–(BUSINESS WIRE)–Ocular Therapeutix™, Inc. (NASDAQ: OCUL), a biopharmaceutical company focused on the formulation, development, and commercialization of innovative therapies for diseases and conditions of the eye, today announced topline results from its Phase 3 clinical trial to evaluate the safety and efficacy of DEXTENZA® for the treatment of ocular itching associated with allergic conjunctivitis (AC). We believe our product DEXTENZA for AC has potential as a hands-free therapy administered in the office setting as a bioresorbable, intracanalicular insert, designed to release the corticosteroid dexamethasone to the ocular surface for up to 30 days. In the Phase 3 trial, DEXTENZA met all pre-specified primary endpoints as demonstrated by a statistically significant mean change in ocular itching from baseline, on a subject-reported 5-point scale, at three time points on Day 8.
“Approximately 10-15 % of my allergic conjunctivitis patients are not adequately controlled with current therapy and thus require use of topical steroids. This patient group comprises commonplace seasonal and perennial allergic conjunctivitis as well as more severe atopic and vernal keratoconjunctivitis”
“We are thrilled with the results from the Phase 3 clinical trial and believe the data highlights a compelling product profile that could potentially change the current standard of care with a one-time, long-acting, hands-free, therapy for the treatment of allergic conjunctivitis,” said Michael Goldstein, MD, Chief Medical Officer. “The allergic conjunctivitis market is currently dominated by topical antihistamine/mast cell stabilizers. There is a large unmet need for a potent, hands-free and preservative-free topical anti-inflammatory medication for those subjects who need more than what it is currently available to them. Even in normal times, the hassle of using topical eye-drops for patients and the worry of non-compliance for physicians are problematic. In the era of COVID-19, having a full course of steroid treatment in a single physician-administered insert that doesn’t require patients to touch their face multiple times per day is an even greater breakthrough.”
“Approximately 10-15 % of my allergic conjunctivitis patients are not adequately controlled with current therapy and thus require use of topical steroids. This patient group comprises commonplace seasonal and perennial allergic conjunctivitis as well as more severe atopic and vernal keratoconjunctivitis,” stated Ken Kenyon, MD, an investigator in the DEXTENZA trial and Clinical Professor of Ophthalmology at Tufts / New England Eye Center. “Having a potent and effective anti-inflammatory medication to personally administer in the office setting without concern for patient compliance or potential misuse would be a great addition to my therapeutic toolbox.”
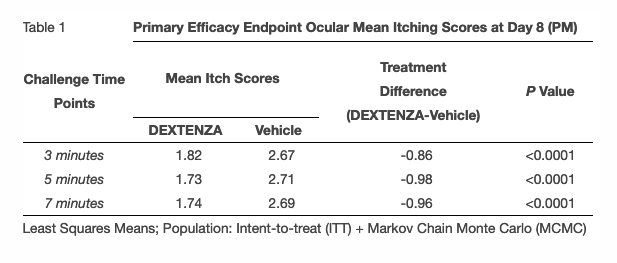
The Phase 3 randomized, double-masked, parallel-arm, placebo-controlled clinical trial enrolled 96 subjects and was conducted across 6 sites in the U.S. using Ora, Inc.’s modified Conjunctival Allergen Challenge (Ora-CAC®) Model. The primary efficacy measure for this trial was ocular itching on Day 8 at 3 minutes, 5 minutes and 7 minutes post-challenge and included subjects with seasonal and perennial allergens. The trial’s primary endpoint was ocular itching measured on a 5-point scale (0 to 4) at three pre-specified time points on Day 8 (PM), 1 week after insertion of DEXTENZA. DEXTENZA-treated subjects demonstrated a statistically significant (p<0.0001) change in ocular itching from baseline at all three pre-specified time points (Table 1). An assessment of the secondary endpoint of ocular itching at all other visits (Day 7, Day 8 (AM), Day 8 (PM, 10 minutes following exposure), Day 14, and Day 15 (AM and PM)) also showed that DEXTENZA-treated subjects had lower itching scores than vehicle-treated subjects at 3 minutes, 5 minutes, 7 minutes and 10 minutes post-CAC (p<.05 for all 21 time points except Day 7 at 3 minutes).
DEXTENZA was observed to have a favorable safety profile and be well-tolerated with no serious adverse events observed (ocular or non-ocular). No subjects required rescue medication and no subjects experienced elevated intraocular pressure. There were 8 ocular treatment emergent adverse events in this trial (2 in the DEXTENZA group and 6 in the vehicle group).
These data are consistent with what has been observed in prior Phase 2 and Phase 3a clinical trials using a similar repeat CAC model. The Company believes that the totality of the efficacy and safety data across the Phase 2 trial and the three Phase 3 trials (n = 323 subjects) represent a compelling package for discussion with the U.S. Food and Drug Administration (FDA) for the treatment of ocular itching associated with AC. This package is also supported by the safety data associated with the prior approval of DEXTENZA for the treatment of inflammation and pain following ophthalmic surgery. The Company will continue to assess additional secondary endpoints over the next several weeks. Upon completion of this review, the Company intends to request a meeting with the FDA to discuss the potential submission of a supplement to its existing new drug application for DEXTENZA to include the treatment of ocular itching associated with AC as an additional approved indication.
“It is estimated that up to 10 million1,2,3 people in the U.S. seek medical attention for the inflammatory response associated with allergic conjunctivitis caused by both seasonal and perennial allergens, representing a discrete and significant potential market for DEXTENZA beyond its current use in the surgical setting,” said Antony Mattessich, the Company’s President and Chief Executive Officer. “In addition to managing the various facets of symptoms associated with AC, we believe that this potential product also addresses physicians’ desire for an abuse-deterrent steroid and patients’ desire for a hands-free solution. From a business perspective, ocular itching associated with AC represents the first indication for DEXTENZA that will be administered in the office setting and is our first potential indication in the large ocular surface disease market. The commercial potential for DEXTENZA in the treatment of ocular surface diseases like AC and episodic dry eye is greater than its potential in the surgical setting.”
Conference Call & Webcast Information
Members of the Ocular Therapeutix management team will host a live conference call and webcast this morning at 8:30 am Eastern Time to discuss the Phase 3 clinical data announcement. The live webcast can be accessed by visiting the Investors section of the Company’s website at investors.ocutx.com. Please connect at least 15 minutes prior to the live webcast to ensure adequate time for any software download that may be needed to access the webcast. Alternatively, please call (844) 464-3934 (U.S.) or (765) 507-2620 (International) to listen to the live conference call. The conference ID number for the live call will be 1460319. An archive of the webcast will be available until July 28, 2020 on the Company’s website.
About DEXTENZA® (dexamethasone ophthalmic insert) 0.4 mg
DEXTENZA is an FDA-approved corticosteroid indicated for the treatment of ocular inflammation and pain following ophthalmic surgery. DEXTENZA is inserted in the lower lacrimal punctum and into the canaliculus by the physician following ophthalmic surgery. A single DEXTENZA releases a 0.4 mg dose of dexamethasone for up to 30 days following insertion. DEXTENZA is preservative free, resorbable and does not require removal.
DEXTENZA is contraindicated in patients with active corneal, conjunctival or canalicular infections. Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, defects in visual acuity and fields of vision. Steroids should be used with caution in the presence of glaucoma. Intraocular pressure should be monitored during the course of the treatment. Corticosteroids may suppress the host response to, and increase the hazard for and severity of, secondary bacterial, viral, or fungal infections. The use of steroids after cataract surgery may delay wound healing and increase the incidence of bleb formation.
The most commonly reported ocular adverse reactions that occurred in patients treated with DEXTENZA were anterior chamber inflammation including iritis and iridocyclitis (10%) and elevations in intraocular pressure (6%). The most common non-ocular adverse reaction was headache (1%).
About Ocular Therapeutix, Inc.
Ocular Therapeutix, Inc. is a biopharmaceutical company focused on the formulation, development, and commercialization of innovative therapies for diseases and conditions of the eye using its proprietary bioresorbable hydrogel-based formulation technology. Ocular Therapeutix’s first commercial drug product, DEXTENZA®, is FDA-approved for the treatment of ocular inflammation and pain following ophthalmic surgery. Ocular Therapeutix has recently completed a Phase 3 clinical trial evaluating DEXTENZA for the treatment of ocular itching associated with allergic conjunctivitis. OTX-TP (intracanalicular travoprost insert) is an intracanalicular insert in clinical development for the reduction of intraocular pressure in patients with primary open-angle glaucoma and ocular hypertension. The Company’s earlier stage assets include OTX-TIC, an extended-delivery intracameral travoprost implant for the reduction of intraocular pressure in patients with glaucoma and ocular hypertension, as well as sustained release intravitreal implants for the treatment of retinal diseases. These intravitreal implants include OTX-TKI, containing the tyrosine kinase inhibitor (TKI) axitinib, and, in collaboration with Regeneron, OTX-IVT, an extended-delivery protein-based anti-vascular endothelial growth factor (VEGF) trap. Ocular Therapeutix’s first product, ReSure® Sealant, is FDA-approved to seal corneal incisions following cataract surgery.
Forward Looking Statements
Any statements in this press release about future expectations, plans, and prospects for the Company, including the commercialization of DEXTENZA®, ReSure Sealant, or any of the Company’s product candidates; the commercial launch of, and effectiveness of reimbursement codes for, DEXTENZA; the development and regulatory status of the Company’s product candidates, such as the Company’s development of and prospects for approvability of DEXTENZA for additional indications including allergic conjunctivitis, OTX-TP for the treatment of primary open-angle glaucoma and ocular hypertension, OTX-TIC for the treatment of primary open-angle glaucoma and ocular hypertension, OTX-TKI for the treatment of retinal diseases including wet AMD, and OTX-IVT as an extended-delivery formulation of the VEGF trap aflibercept for the treatment of retinal diseases including wet AMD; the ongoing development of the Company’s extended-delivery hydrogel depot technology; the potential utility of any of the Company’s product candidates; the potential benefits and future operation of the collaboration with Regeneron Pharmaceuticals, including any potential future payments thereunder; the expected impact of the COVID-19 pandemic on the Company and its operations; the sufficiency of the Company’s cash resources and other statements containing the words “anticipate,” “believe,” “estimate,” “expect,” “intend”, “goal,” “may”, “might,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors. Such forward-looking statements involve substantial risks and uncertainties that could cause the Company’s clinical development programs, future results, performance or achievements to differ significantly from those expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, the timing and costs involved in commercializing DEXTENZA, ReSure Sealant or any product candidate that receives regulatory approval, including the conduct of post-approval studies, the ability to retain regulatory approval of DEXTENZA, ReSure Sealant or any product candidate that receives regulatory approval, the ability to maintain reimbursement codes for DEXTENZA, the initiation, timing and conduct of clinical trials, availability of data from clinical trials and expectations for regulatory submissions and approvals, the Company’s scientific approach and general development progress, the availability or commercial potential of the Company’s product candidates, the Company’s ability to generate its projected net product revenue on the timeline expected, if at all, the sufficiency of cash resources, the Company’s existing indebtedness, the ability of the Company’s creditors to accelerate the maturity of such indebtedness upon the occurrence of certain events of default, the outcome of the Company’s ongoing legal proceedings, the severity and duration of the COVID-19 pandemic including its effect on the Company’s and relevant regulatory authorities’ operations, the need for additional financing or other actions and other factors discussed in the “Risk Factors” section contained in the Company’s quarterly and annual reports on file with the Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent the Company’s views as of the date of this release. The Company anticipates that subsequent events and developments will cause the Company’s views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, the Company specifically disclaims any obligation to do so except as required by law. These forward-looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date of this release.
1 Leonardi A, Castegnaro A, Valerio ALG, Lazzarini D. Epidemiology of allergic conjunctivitis: clinical appearance and treatment patterns in a population-based study. Curr Opin Allergy Clin Immunol. 2015;15(5):482-488.
2 Rosario N, Bielory L. Epidemiology of allergic conjunctivitis. Curr Opin Allergy Clin Immunol. 2011;11(5):471-476
3 Ora website, An Update on Ocular Allergy Trends, 2019 Ora, Inc., www.oraclinical.com
Investors
Ocular Therapeutix
Donald Notman
Chief Financial Officer
dnotman@ocutx.com
or
Westwicke, an ICR Company
Chris Brinzey, 339-970-2843
Managing Director
chris.brinzey@westwicke.com
Media
Ocular Therapeutix
Scott Corning
Senior Vice President, Commercial
scorning@ocutx.com