Hover over the tiles to learn more.










Hover over map to learn more.
Hover over the tiles to learn more.
Extensive experience supporting our customers across various indications
Extensive experience supporting our customers across various indications including:
Dry Eye: 30 therapies evaluated including 2 most recent, novel approvals: Xiidra and TrueTear
Allergy: Supported partners in the approval of 23 products
Industry leader in IRDs, Wet/Dry AMD, and Glaucoma
Industry leader in IRDs, Wet/Dry AMD, and Glaucoma
Advancing research with innovative capabilities and novel endpoints
Retina R&D Team dedicated to clinical model and endpoint development
Global clinical and regulatory experience at all levels of research
Global clinical and regulatory experience at all levels of research in areas including IOLs, OCT, and Contact Lenses
30+ FDA pre-submissions
10+ PMA/HDE
20+ 510(k)
In recent years
Click the buttons below to learn about each indication’s totals from
the last 5 years.

Click on each tab to learn more.


















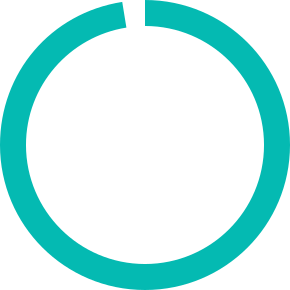
of Clinical Project Managers have 5+ years of experience in ophthalmology
Click on the tiles to learn more.
Upholding the highest standards of data quality, integrity, and security
Upholding the highest standards of data quality, integrity, and security
Experts in designing and analyzing ophthalmic clinical trials
Experts in designing and analyzing ophthalmic clinical trials
Interactive Response Technology (IRT) solutions for complex study needs
Interactive Response Technology (IRT) solutions for complex study needs

